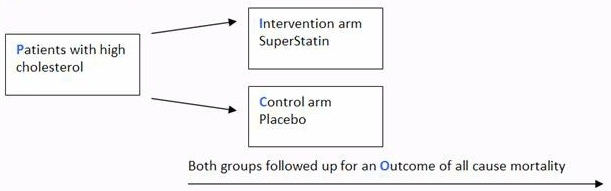
The odds ratio is calculated using the number of case patients who did or did not have exposure to a factor (such as a particular food) and the number of controls who did or did no t have the exposure The odds ratio tells us how much higher the odds of exposure are among casepatients than among controls An odds ratio of • 10 (or close to 10) The basic difference is that the odds ratio is a ratio of two odds (yep, it's that obvious) whereas the relative risk is a ratio of two probabilities (The relative risk is also called the risk ratio) Let's look at an example Relative Risk/Risk Ratio Suppose you have a school that wants to test out a new tutoring program The Odds Ratio The relative exposure distributions (7/6) and (10/56) are really odds, ie the odds of exposure among cases and nondiseased controlsIf we compute the ratio of these two odds we would get ie, almost identical to the risk ratio we calculated when we had all the information for the source group

On Biostatistics And Clinical Trials Odds Ratio And Relative Risk
Odds ratio vs relative risk cohort
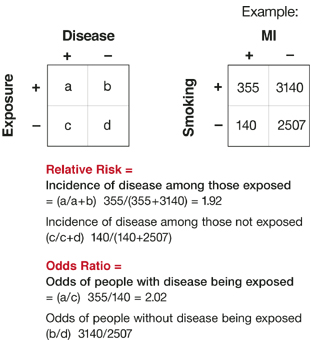
Odds ratio vs relative risk cohort- The relative risk is the ratio of the risk in the exposed group to the risk in the unexposed group, as is summarized in Box 1 Depending on the study design and statistical method applied, the relative risk can be presented using different measures of effect, such as the incidence rate ratio and hazard ratioOdds of the disease in men Odds=Risk of disease in men/risk of no disease in men= (140/0)/(60/0)=07




Comparison Of Three Methods For Estimating Relative Risk In A Cohort Download Table
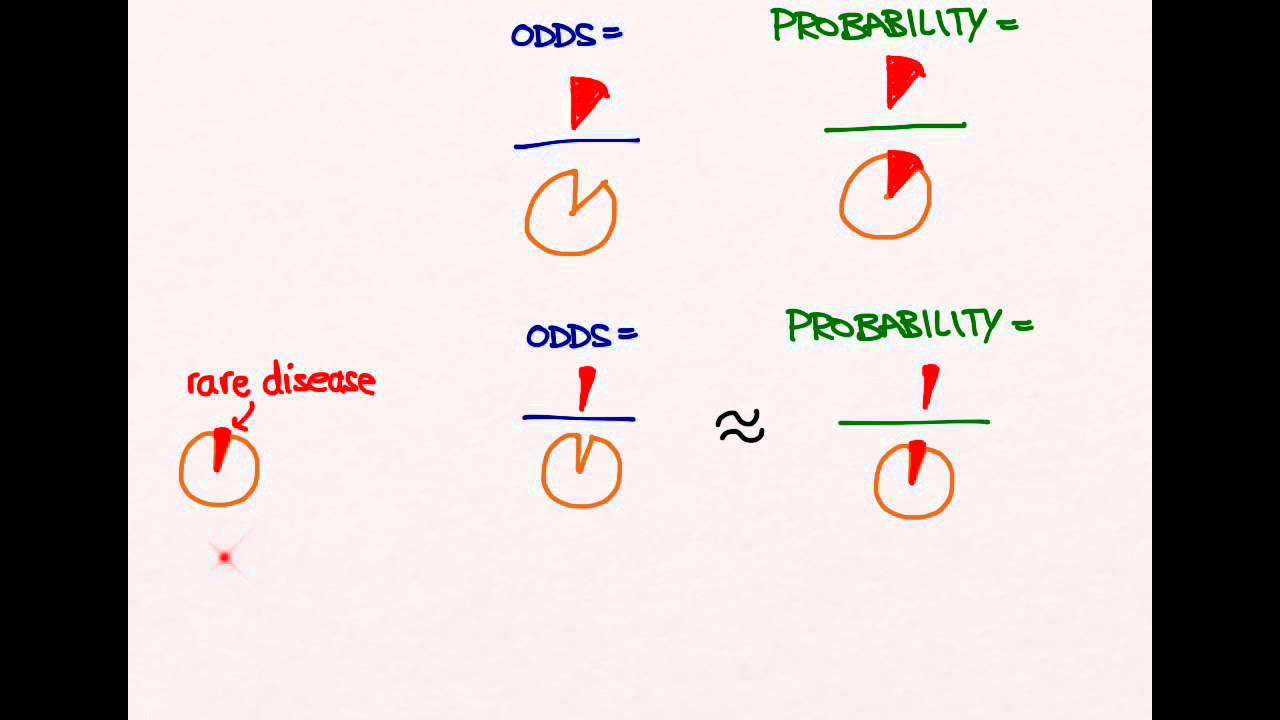
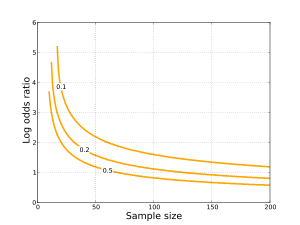
Hi, Been reading through a research paper that used a prospective cohort study, but in the results table for measures of association, the odds ratio was used instead of relative risk Subsequently, the term relative risk commonly refers to either the risk ratio or the odds ratio However, only under certain conditions does the odds ratio approximate the risk ratio Figure 1 shows that when the incidence of an outcome of interest in the study population is low (Odds Ratio (OR) is a ratio or proportion of odds I just remember that odds ratio is a ratio of odds and probability isn't a ratio of odds (AKA it is the other option) Relative Risk = Probability / Probability Odds Ratio = Odds / Odds Now that you have a general idea of what odds ratio and relative risk are you need to know when to use
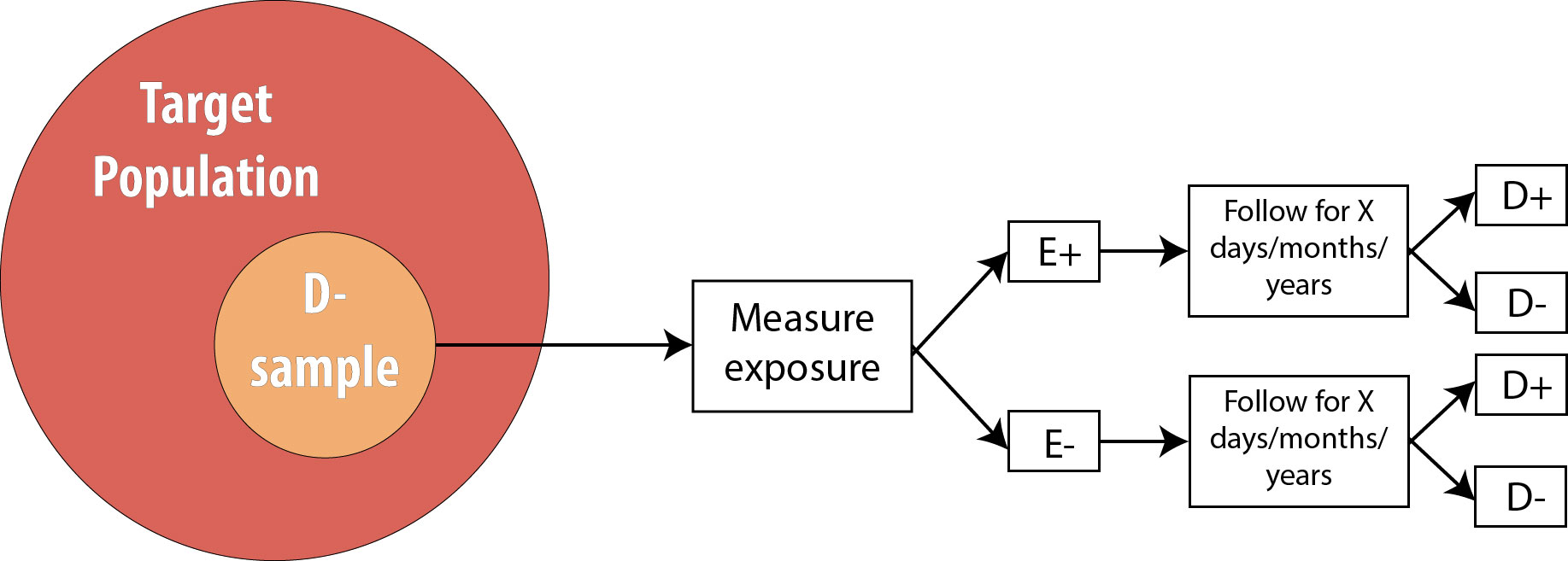
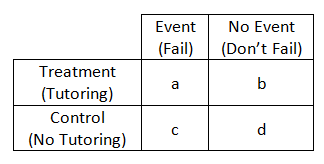
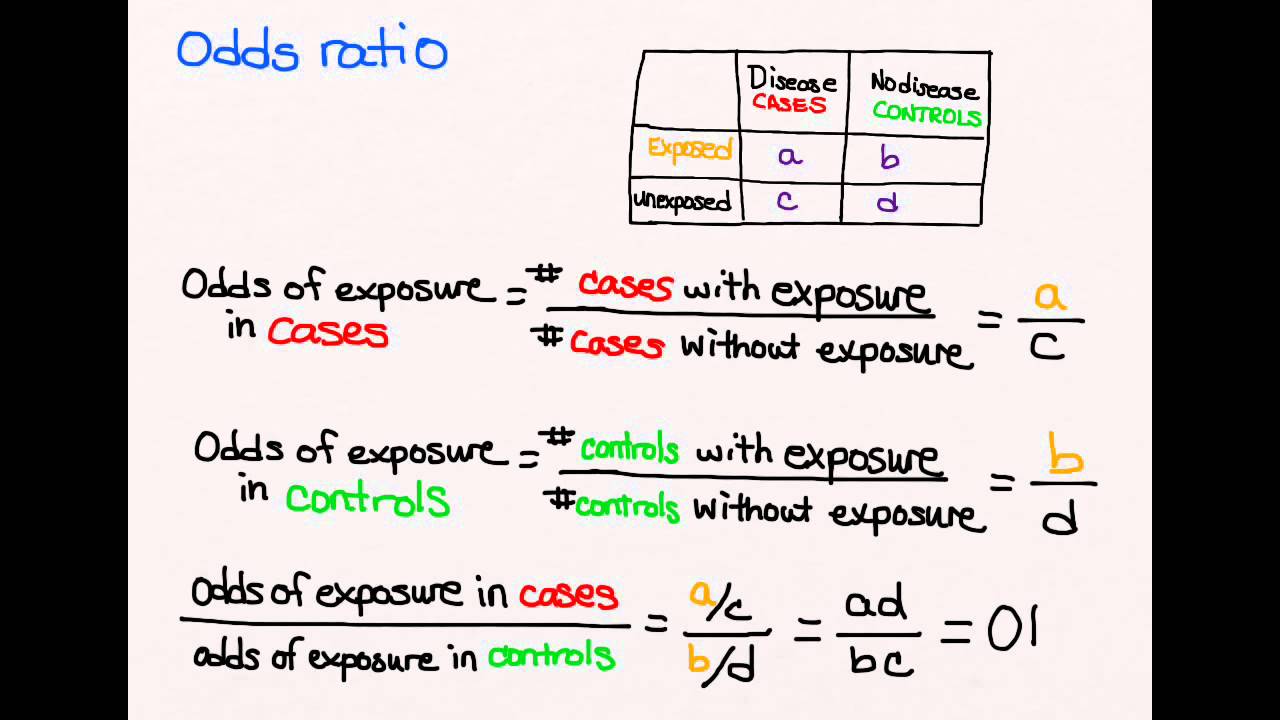
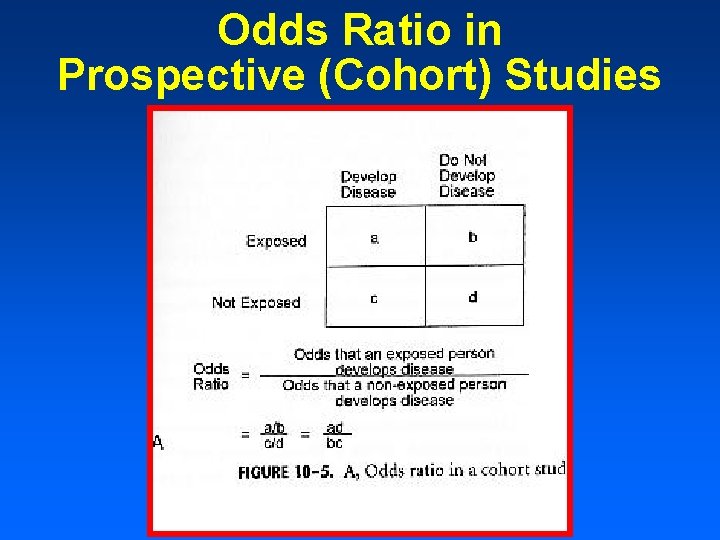
Case–control study versus cohort on a timeline "OR" stands for "odds ratio" and "RR" stands for "relative risk" A retrospective cohort study, also called a historic cohort study, is a longitudinal cohort study used in medical and psychological research A cohort of individuals that share a common exposure factor is compared with anotherPressed as the risk ratio in cohort studiesandclinicaltrialsWhentheriskratio cannot be obtained directly (such as in a casecontrolstudy),theoddsratioiscalculated and often interpreted as if it were the risk ratio Subsequently, the term relative risk commonly refers to either the risk ratio or the odds ratio However, only under certain The odds ratio (OR) is the ratio of the odds of cancer in smokers to the odds of cancer in nonsmokers OR = (a/b)/ (c/d) = (ad)/ (bc) The risk ratio (RR), also called the relative risk, is the ratio of the probability of cancer in smokers to the probability of cancer in nonsmokers Given that you know a, b, c, and d, you can compute either of
A crude odds ratio can be converted to a crude risk ratio risk ratio = odds ratio/(1 − p0) (p0 × odds ratio), in which p0 is the outcome prevalence (risk) among the unexposed Some have applied this formula to an adjusted odds ratio to obtain an adjusted risk ratio 49 This method can produce biased risk ratios and incorrect confidence intervals 26 , 32 Davies et al (1) state that the odds ratio is a common measure in casecontrol studies, cohort studies, or clinical trails Unfortunately, this first sentence of their article is not correct For different study designs, OR should only be used as a measure of effect size when RR can not be estimated directlyComes can often be summarized by estimating odds ratios or risk ratios If the study outcome is sufficiently rare among exposed and unexposed study subjects, the odds ratio for the exposure–outcome association will closely approximate the risk ratio But if the outcome is common and the risk ratio is not close to 1, the odds ratio will be




How To Be Awesome At Biostatistics And Literature Evaluation Part Ii Tl Dr Pharmacy




Relative Risk And Odds Ratio
Relative Risk and Odds Ratio The Relative Risk (RR) is used to compare the probability of an event between two different groups It is simply the ratio of the probability of the event occurring in two, mutually exclusive groups RR = π1 / π2 A RR of 1 means there is no difference in risk Odds ratio can be calculated in a cohort study and in a casecontrol study The exposure odds ratio is equal to the disease odds ratio Relative risk can only be calculated in a cohort study Odds ratio can be a measure of relative risk in case control study 6The relative risk (also known as risk ratio RR) is the ratio of risk of an event in one group (eg, exposed group) versus the risk of the event in the other group (eg, nonexposed group) The odds ratio (OR) is the ratio of odds of an event in one group versus the odds of the event in the other group




Measures Of Effect Relative Risks Odds Ratios Risk Difference And Number Needed To Treat Kidney International




Believability Of Relative Risks And Odds Ratios In Abstracts Cross Sectional Study The Bmj
Relative risks and odds ratios are widely reported in the medical literature, but can be very difficult to understand We sought to further clarify these important indices Methods We illustrated both relative risks and odds ratios using bar charts, then looked at the types of study for which each statistic is suitedIn practice the odds ratio is commonly used for casecontrol studies, as the relative risk cannot be estimated In fact, the odds ratio has much more common use in statistics, since logistic regression, often associated with clinical trials, works with the log of the odds ratio, not relative riskThe risk of getting the disease in males is 31 times the risk of getting the disease in females •What is the odds ratio for the disease among men as opposed to women?



Odds Ratios And Risk Ratios Youtube



Interpretation Of Genetic Association Studies Markers With Replicated Highly Significant Odds Ratios May Be Poor Classifiers
Given that this is a cohort study, the relative risk (RR) should have been calculated instead of the odds ratio Odds ratios frequently generate larger differences between exposed and nonexposed groups (especially when dealing with nonrare events), therefore overestimating the observed effect (4) INTRODUCTION Odds ratio (OR) and risk ratio (RR) are two commonly used measures of association reported in research studies In crosssectional studies, the odds ratio is also referred to as the prevalence odds ratio (POR) when prevalent cases are included, and, instead of the RR, the prevalence ratio (PR) is calculatedThe quote surely just means to say that the odds ratio is a relative risk measure rather than an estimate of the relative risk, which as already point out is only approximately the case in cohort studies/randomized trials for very low proportions By relative risk measure I mean something that is given relative to some comparison group in a way that the absolute difference depends on the



Relative Risks And Odds Ratios What S The Difference Mdedge Family Medicine



Individual And Community Level Risk For Covid 19 Mortality In The United States Nature Medicine
Odds Ratio, Relative Risk, and Risk Difference How to Use Odds Ratio, Relative Risk, and Risk Difference to Describe the Association Between Two CategoricalAbstract Odds ratios (OR) are commonly reported in the medical literature as the measure of association between exposure and outcome However, it is relative risk that people more intuitively understand as a measure of association Relative risk can be directly determined in a cohort study by calculating a risk ratio (RR)When used for cohort studies and randomized clinical trials, the odds ratio is often incorrectly interpreted as the risk ratio;



Http Osctr Ouhsc Edu Sites Default Files 02 Module7partinotes Pdf




Cureus What S The Risk Differentiating Risk Ratios Odds Ratios And Hazard Ratios
Nonexposed group, "OR" is an odds ratio from a logistic regression equation, and "RR" is an estimated relative risk Most researchers apply this formula to the adjusted odds ratio to estimate an adjusted relative risk Using the formula in this manner is incorrect and will produce a biased estimate when confounding is present If the risk ratio is 1 (or close to 1), it suggests no difference or little difference in risk (incidence in each group is the same) A risk ratio > 1 suggests an increased risk of that outcome in the exposed group A risk ratio < 1 suggests a reduced risk in the exposed group Percent RelativeMay be correctly applied to casecontrol studies, in cohort studies we are often interested in estimating a relative risk (or, rate ratio), not the odds ratio In studies of common outcomes, the estimated odds ratio can substantially overestimate the relative risk Regardless the difference between an odds ratio and a relative risk, authors and




Relative And Absolute Risk Osmosis



1
–45 5 McNutt L, Wu C, Xue X, Hafner JP Estimating the relative risk in cohort studies and clinical trials of common outcomes Am J Epidemiol 03;The relative merits of risk ratios and odds ratios Arch Pediatr Adolesc Med 09;In epidemiological terms, the odds ratio is used as a point estimate of the relative risk in retrospective studies Odds ratio is the key statistic for most casecontrol studies In prospective studies, Attributable risk or risk difference is used to quantify risk in the exposed group that is attributable to the exposure In retrospective studies, attributable risk can not be calculated



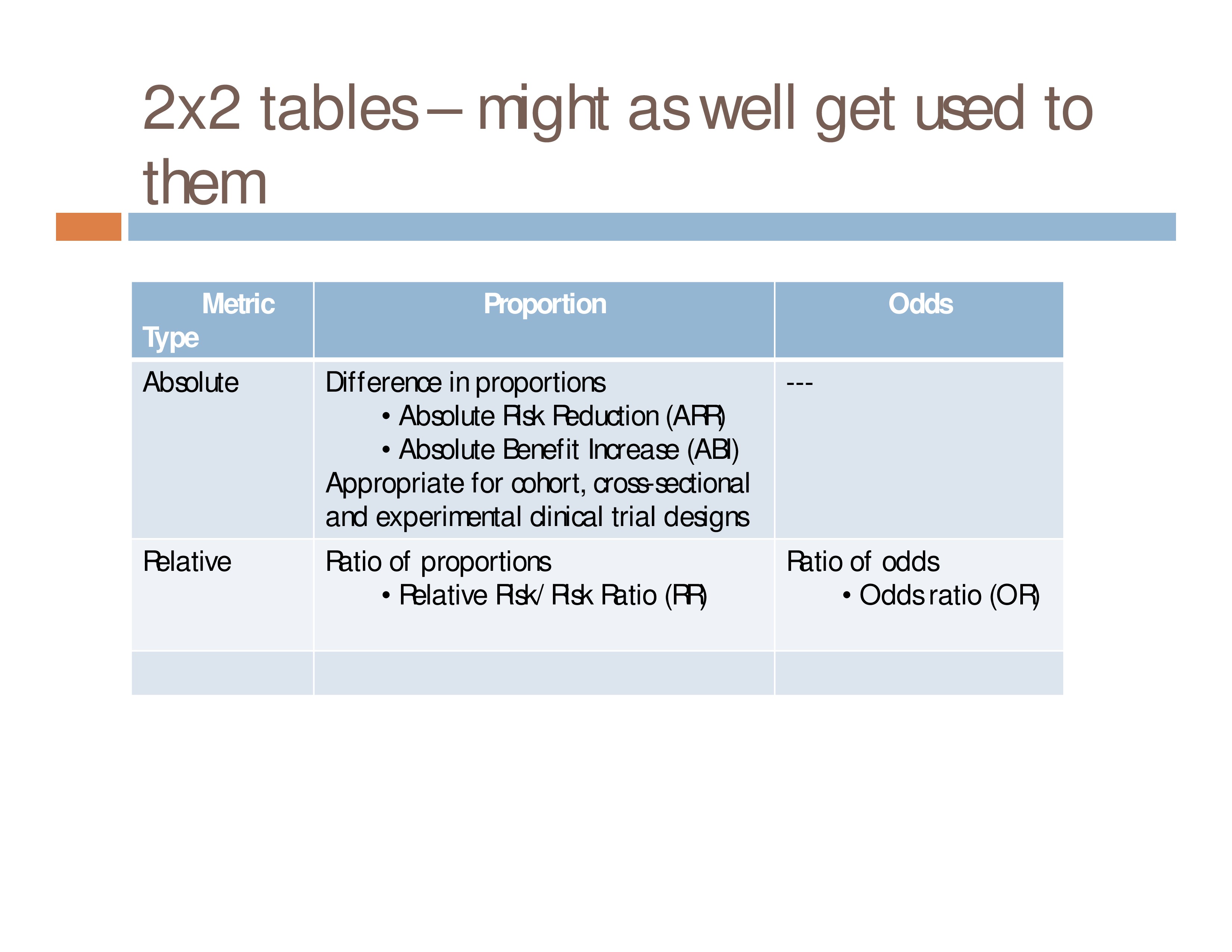
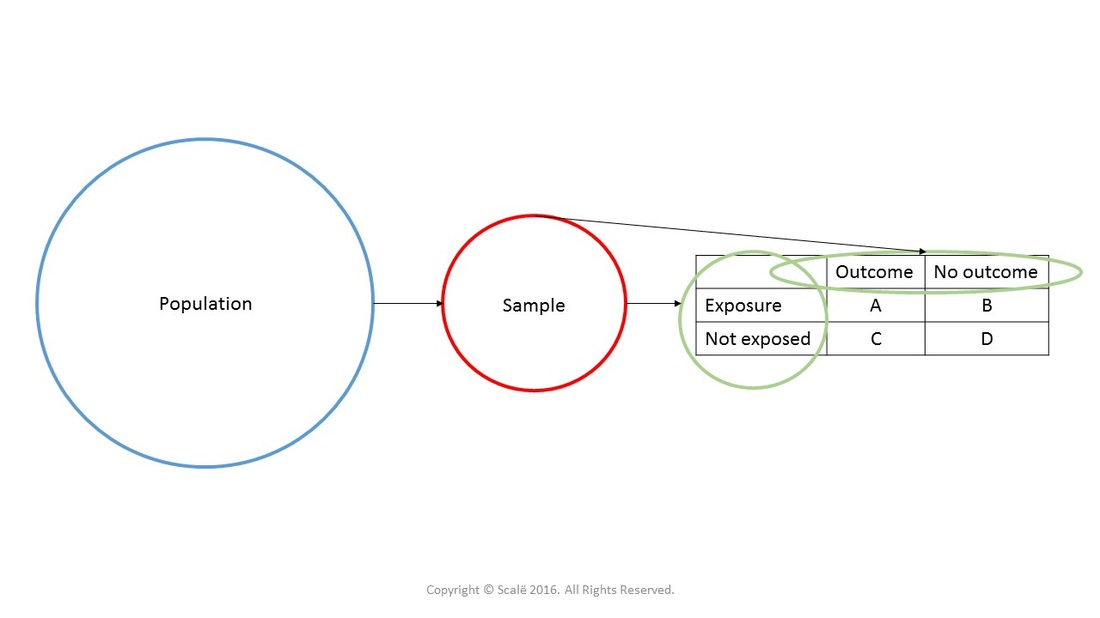
Introduction To 2 X 2 Tables Epidemiologic Study Design And Measures Of Association Foundations Of Epidemiology
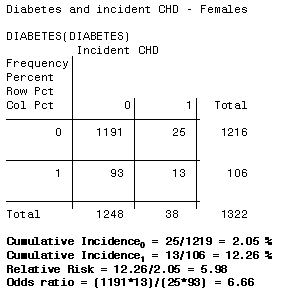



3 5 Bias Confounding And Effect Modification Stat 507
Odds ratios are a common measure of the size of an effect and may be reported in casecontrol studies, cohort studies, or clinical trials Increasingly, they are also used to report the findings from systematic reviews and metaanalyses Odds ratios are hard to comprehend directly and are usually interpreted as being equivalent to the relative risk• Odds ratio can be calculated in a cohort study and a casecontrol study • Relative risk can only be calculated in a cohort study Compare the incidence of developing new cases A cohort study follows patients with risk factor (or exposure) until the Here's the key Relative Risk looks to the future for the effect of a particular cause hence it is used in prospective studies say a cohort study Lets compare the above with Odds Ratio The Odds Ratio can be addressed by asking te following question How many times more likely is a diseased group to have been exposed to a risk factor as




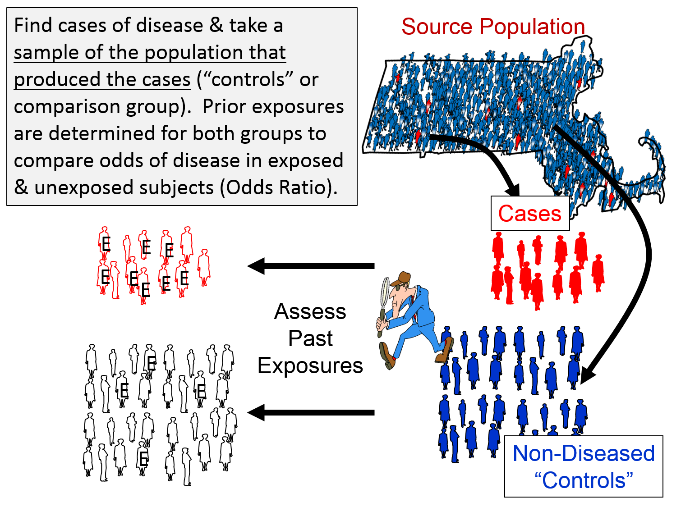
A Practical Overview Of Case Control Studies In Clinical Practice Chest



Case Control Study Vs Cohort Study Pp Made Easy On Vimeo
The term "relative risk (RR)" has been traditionally used to convey the relative probability calculations in both cohort (for incidence ratios) and crosssectional studies (for prevalence ratiosAbout Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How works Test new features Press Copyright Contact us CreatorsThe relative risk is nobler than the odds ratio If the prevalence is higher, the relative risk is used
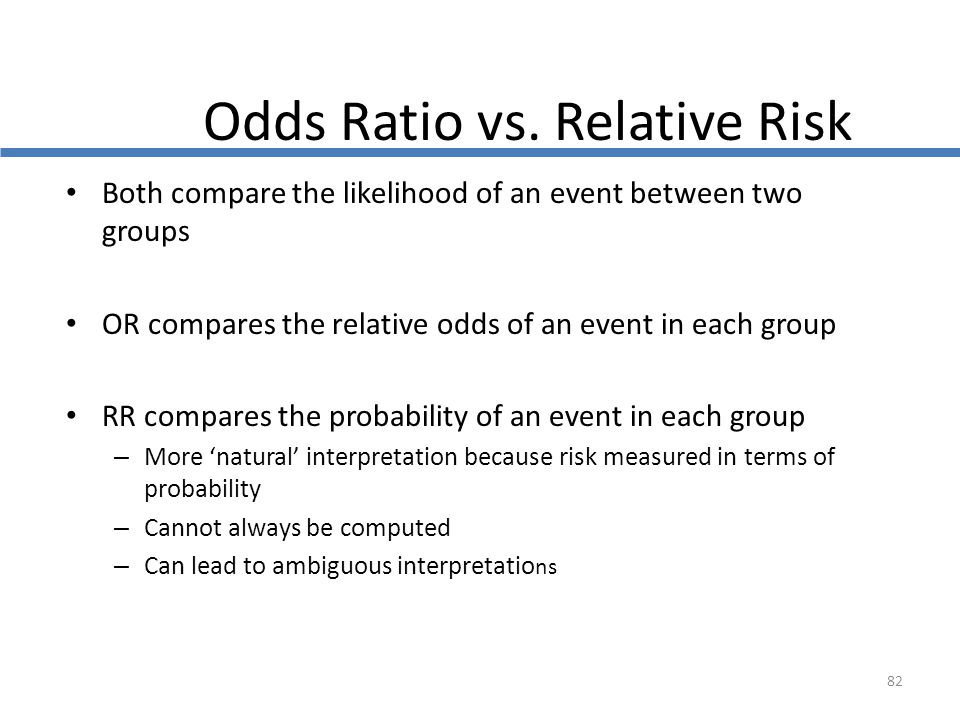



Cph Exam Review Epidemiology Ppt Download
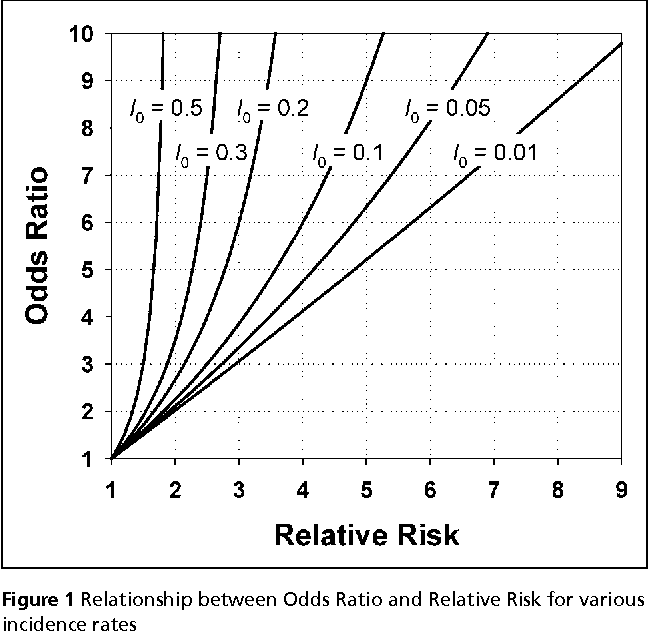



Pdf When To Use The Odds Ratio Or The Relative Risk Semantic Scholar
One of the most commonly observational study designs employed in veterinary is the crosssectional study with binary outcomes To measure an association with exposure, the use of prevalence ratios (PR) or odds ratios (OR) are possible In human epidemiology, much has been discussed about the use of the OR exclusively for case–control studies and some authorsThe odds ratio then provides an overestimation of the risk ratio, especially when the outcome is frequent The use of logistic regression to adjust for confounding is one of the reasons that odds ratios are presented Definition of risk ratio A risk ratio (RR), also called relative risk, compares the risk of a health event (disease, injury, risk factor, or death) among one group with the risk among another group It does so by dividing the risk (incidence proportion, attack rate) in group 1 by the risk (incidence proportion, attack rate) in group 2



Odds Vs Relative Risk ただの悪魔の画像




A Most Odd Ratio Interpreting And Describing Odds Ratios Abstract Europe Pmc
Converting Odds Ratio to Relative Risk in Cohort Studies with Partial Data Information Zhu Wang Connecticut Children's Medical Center Abstract In medical and epidemiological studies, the odds ratio is a commonly applied measure to approximate the relative risk or risk ratio in cohort studies It is well known tha such It is assumed that, if the prevalence of the disease is low, then the odds ratio approaches the relative risk Case control studies are relatively inexpensive and less timeconsuming than cohort studies In this case the odds ratio (OR) is equal to 16 and the relative risk (RR) is equal to 865 The measure of association between exposure and disease in cohort studies is the relative risk The relative risk is the ratio of the incidence rate of index subjects to that of control subjects A relative risk of 10 signifies that the incidence rate is the same among exposed and nonexposed subjects and indicates a lack of association between exposure and disease




最新 Odds Ratio Vs Relative Risk Usmle ただの悪魔の画像



Definition And Calculation Of Odds Ratio Relative Risk Stomp On Step1
Risk ratios, odds ratios, and hazard ratios are three ubiquitous statistical measures in clinical research, yet are often misused or misunderstood in their interpretation of a study's results A 01 paper looking at the use of odds ratios in obstetrics and gynecology research reported 26% of studies (N = 151) misinterpreted odds ratios as risk ratios , while aOdds ratios and risk ratios •How do you interpret the relative risk?Percent, population attributable risk percent, relative risk, odds, odds ratio, and others The concept and method of calculation are explained for each of these in simple terms and with the help of examples The interpretation of each is presented in plain English rather than in technical language Clinically useful notes are provided,




A Most Odd Ratio American Journal Of Preventive Medicine




Relative Risk Odds Ratios Youtube




Cohort And Case Con Revised



Number Needed To Treat




Statistics Part 13 Measuring Association Between Categorical Data Relative Risk Odds Ratio Attributable Risk Logistic Regression Data Lab Bangladesh




Comparison Of Three Methods For Estimating Relative Risk In A Cohort Download Table



A Beginner S Guide To Interpreting Odds Ratios Confidence Intervals And P Values Students 4 Best Evidence



Relative Risk Wikipedia



Odds Ratio Wikipedia




How To Use Spss For Contingency Table Relative Risk Odds Ratio




1 Relative Risks Odds Ratios Or Hazard Ratios Of Risk Factors For Download Table



The Difference Between Relative Risk And Odds Ratios The Analysis Factor



Relative Risk Odds Ratio



Event Based Measures Of Effect Size Asha Journals Academy



Frontiers Odds Ratio Or Prevalence Ratio An Overview Of Reported Statistical Methods And Appropriateness Of Interpretations In Cross Sectional Studies With Dichotomous Outcomes In Veterinary Medicine Veterinary Science




On Biostatistics And Clinical Trials Odds Ratio And Relative Risk




Critical Numbers Living With Risk At The End
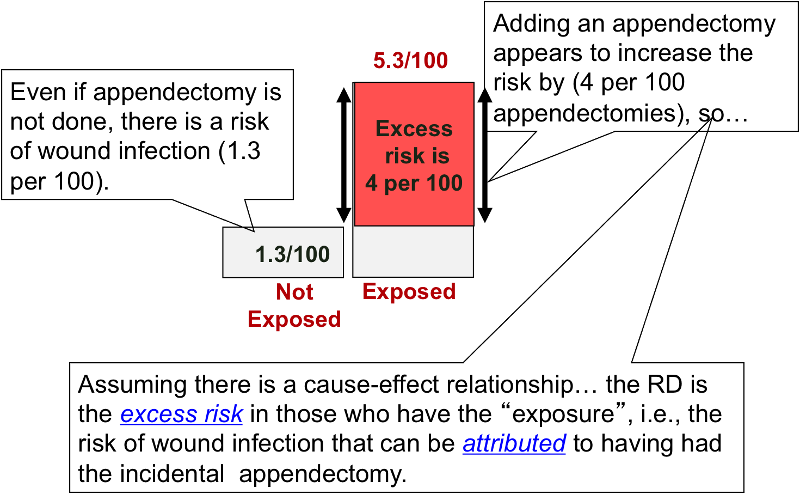



Risk Differences And Rate Differences




Relative Risk Wikipedia




Statistics For Afp Dr Mohammad A Fallaha Afp



Ppt Odds Ratio Vs Relative Risk Powerpoint Presentation Free Download Id




Relative Risk Or Odds Ratio For Cardiovascular Disease Incidence Download Scientific Diagram
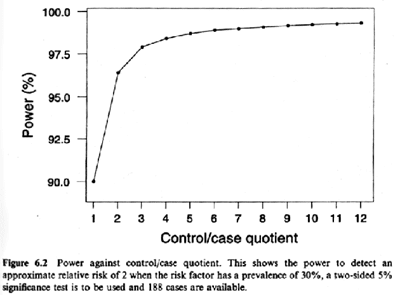



Lesson 9 Cohort Study Design Sample Size And Power Considerations For Epidemiologic Studies
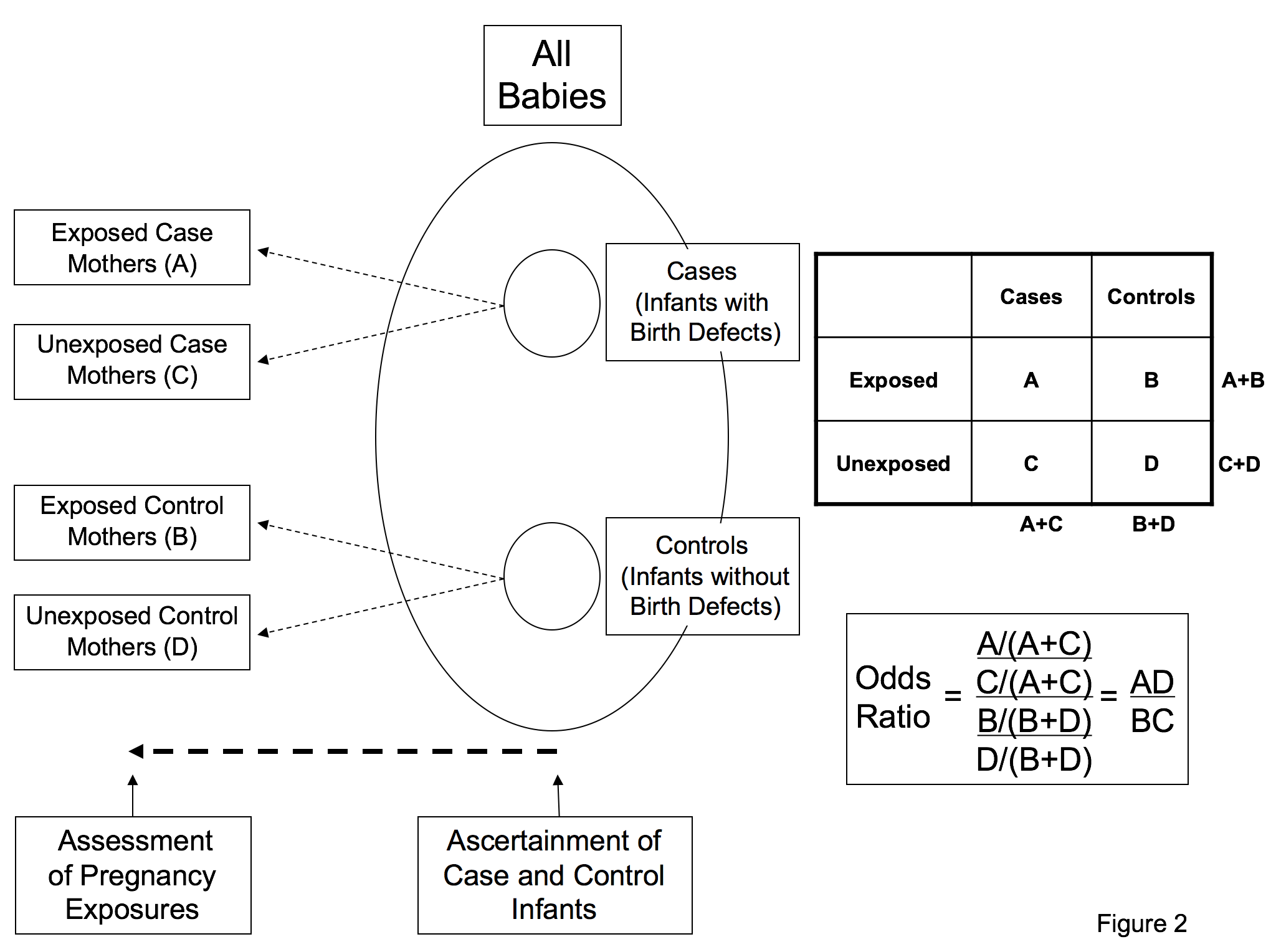



Society For Birth Defects Research And Prevention



Research Statistics Basics Contents 1 Basic Concepts 2 References Basic Concepts Null Hypothesis The Hypothesis That The Independent Variable Has No Effect On The Dependent Variable For Example Steroids Do Not Improve Outcomes In Ards Would Be




Forest Plot Of Relative Risks And Odds Ratios Of Lung Cancer Associated Download Scientific Diagram
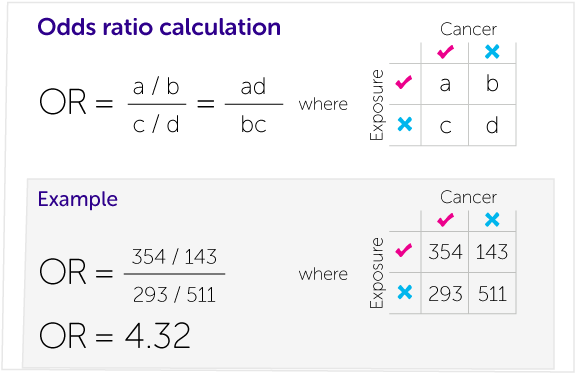



Our Calculations Explained Cancer Research Uk




Research Techniques Made Simple Interpreting Measures Of Association In Clinical Research Sciencedirect
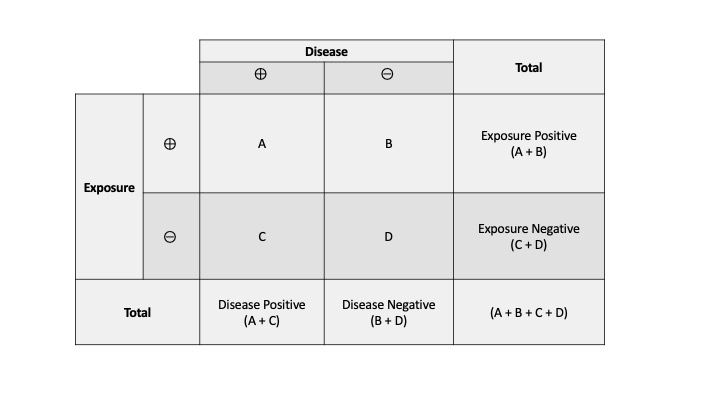



Measures Of Association Stats Medbullets Step 1
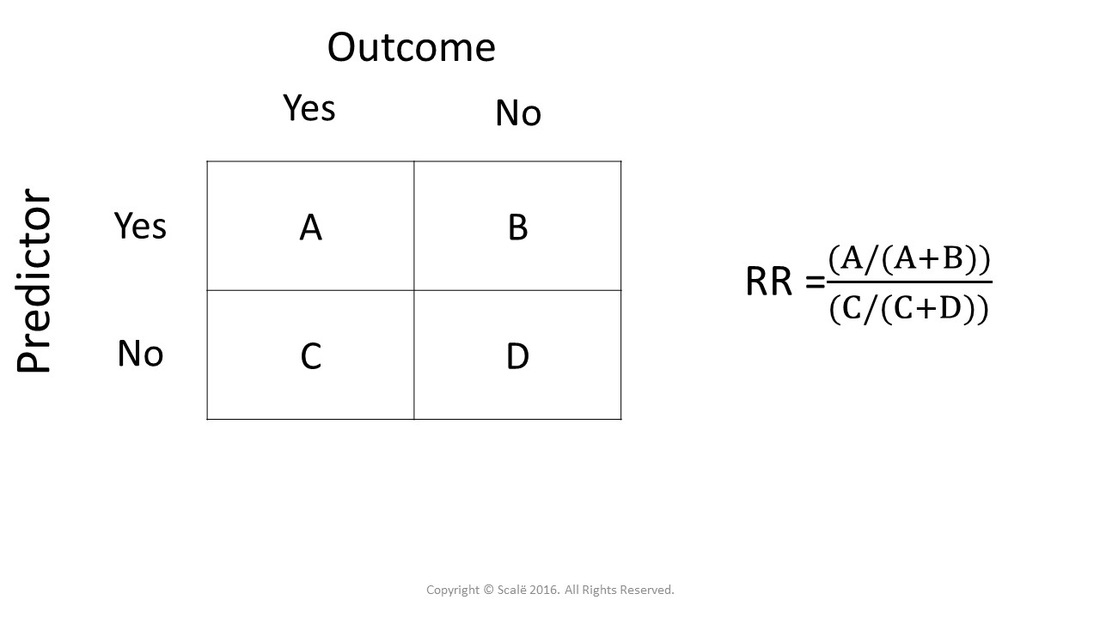



Calculate Relative Risk With 95 Confidence Intervals



Relative Risk Ratios And Odds Ratios




Definition And Calculation Of Odds Ratio Relative Risk Stomp On Step1




On Biostatistics And Clinical Trials Odds Ratio And Relative Risk
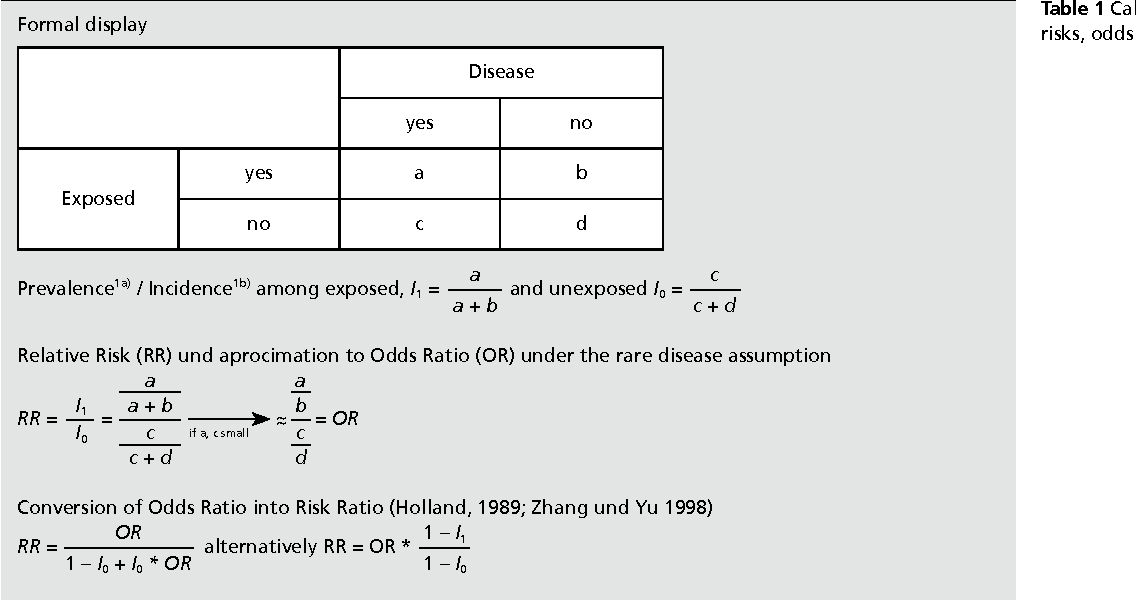



Pdf When To Use The Odds Ratio Or The Relative Risk Semantic Scholar




Relative Risk And Odds Ratio Usmle The Journey




Odds Ratio Wikipedia



Silo Tips Download Transcript Measuring Risk In Epidemiology B D A C Measuring Risk In Epidemiology
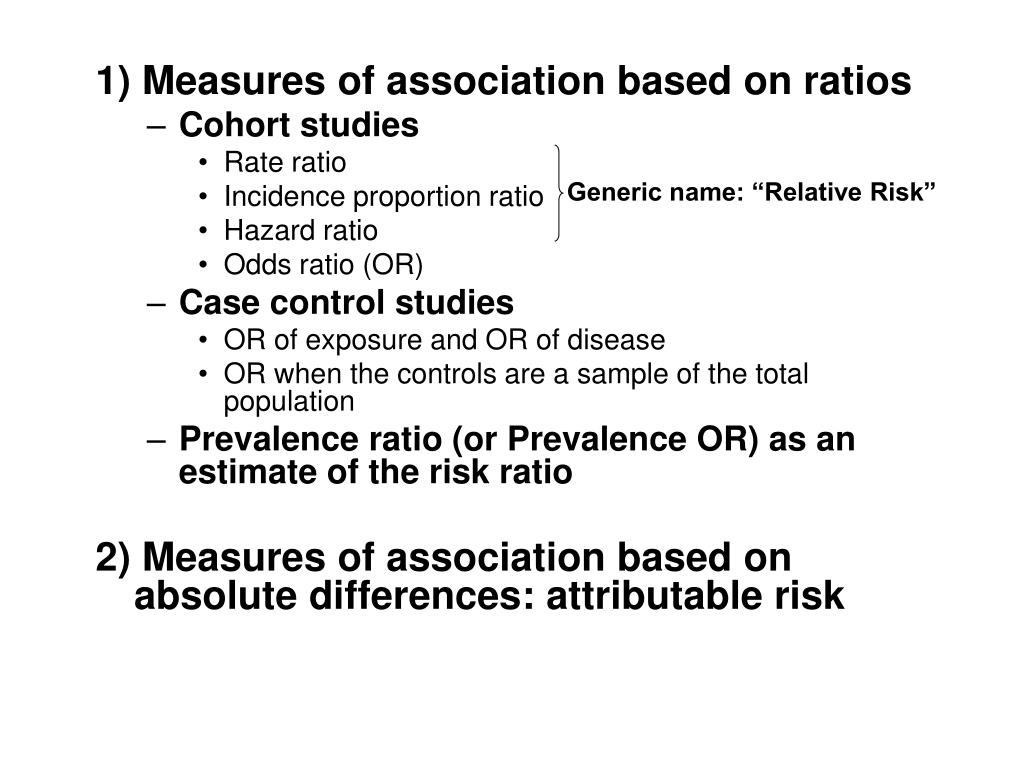



Ppt Measures Of Association Powerpoint Presentation Free Download Id




Odds Ratio Relative Risk Calculation Definition Probability Odds Youtube
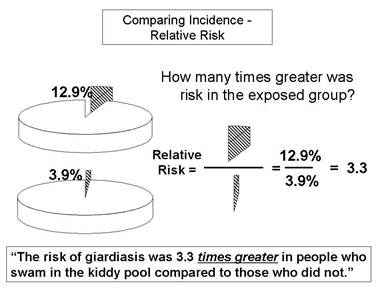



Relative Risk And Absolute Risk Definition And Examples Statistics How To




Relative Risk Versus Odds Ratio Usmle Biostatistics 4 Youtube



Retrospective Cohort Study Wikipedia



Main Points To Be Covered Measures Of Association In Case Control Studies Prevalent Controls Design Odds Ratio Vs Relative Risk And The Rare Disease Ppt Download




Measures Of Association Ppt Download



9 10 11 12 13 14 15 16 17 18 19 21 22 23 24 25 26 27 28 29 30 Review Odds Ratios Are Calculated From Case Control Studies Which Are Described On Slide 14 Odds Ratios Are Only Estimates Of Relative Risks Since True Incidence Rates Cannot Be



Attributable




Abdullah Kharbosh What Does An Odds Ratio Or Relative Risk Mean By Ebmteacher Casecontrol Cohort T Co Shfiaepl57 عبر Slideshare



Escholarship Umassmed Edu Cgi Viewcontent Cgi Article 1013 Context Liberia Peer




1 The Odds Ratio Relative Odds In A Case Control Study We Do Not Know The Incidence In The Exposed Population Or The Incidence In The Nonexposed Population Ppt Download
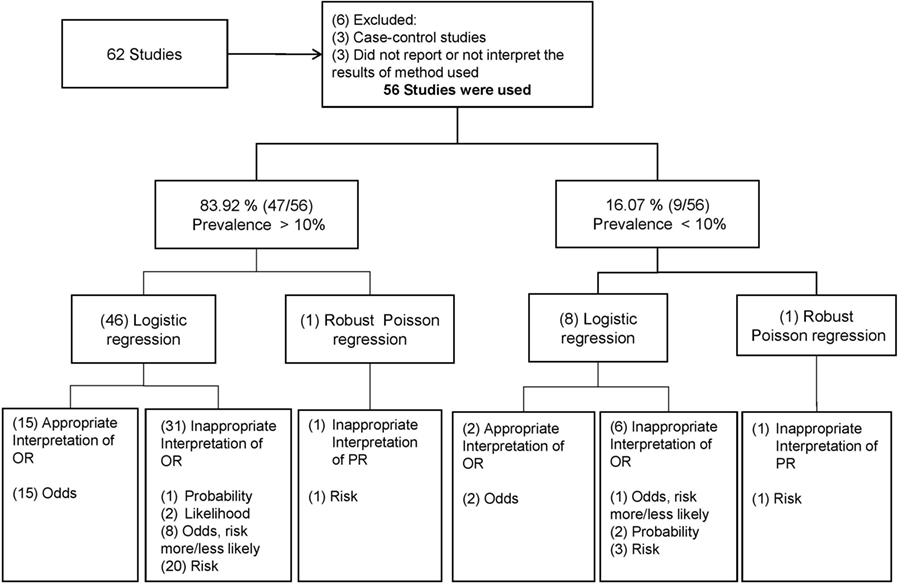



Frontiers Odds Ratio Or Prevalence Ratio An Overview Of Reported Statistical Methods And Appropriateness Of Interpretations In Cross Sectional Studies With Dichotomous Outcomes In Veterinary Medicine Veterinary Science



Silo Tips Download Transcript Measuring Risk In Epidemiology B D A C Measuring Risk In Epidemiology
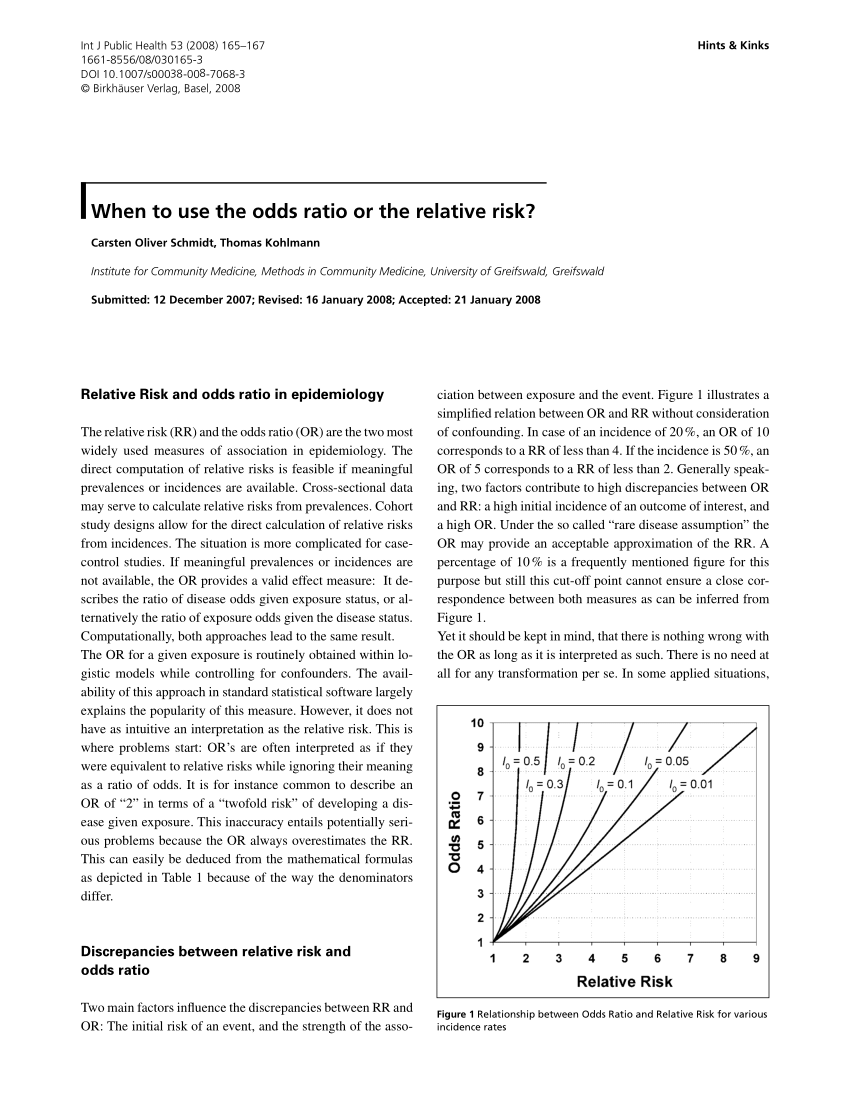



Pdf When To Use The Odds Ratio Or The Relative Risk



Beaumont Cloud Cme Com Launchscorm Aspx Caseid 112 Userid 0 Video True



Calculate Relative Risk With 95 Confidence Intervals



Using Odds Ratio In Case Control Studies Youtube



A Nested Case Control Study



Epidemiology Knowledge Amboss



How To Interpret And Use A Relative Risk And An Odds Ratio Youtube



Risk Ratio




When Can Odds Ratios Mislead The Bmj
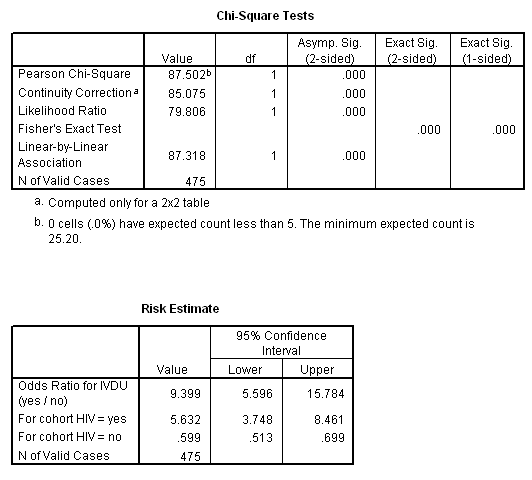



Marg Innovera




Fully Adjusted Odds Ratio Or Relative Risk For Hypertension Compared Download Scientific Diagram
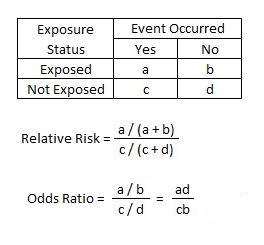



Relative Risk Article



Risk Ratio Vs Odds Ratio Hunter 19 Notes And Things



Odds Ratios Need To Be Graphed On Log Scales Andrew Wheeler




Relative And Attributable Risks Absolute Risk Involves People




Measures Of Effect Relative Risks Odds Ratios Risk Difference And Number Needed To Treat Kidney International




Estimating Risk
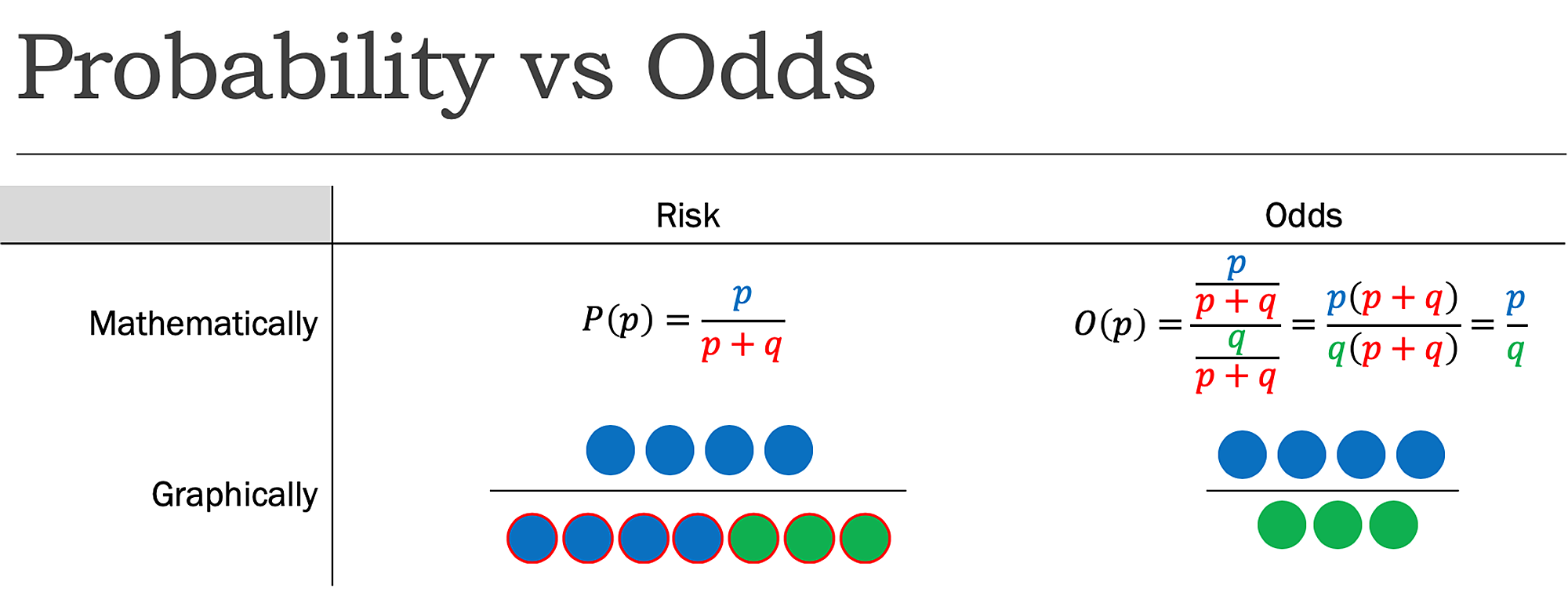



Cureus What S The Risk Differentiating Risk Ratios Odds Ratios And Hazard Ratios
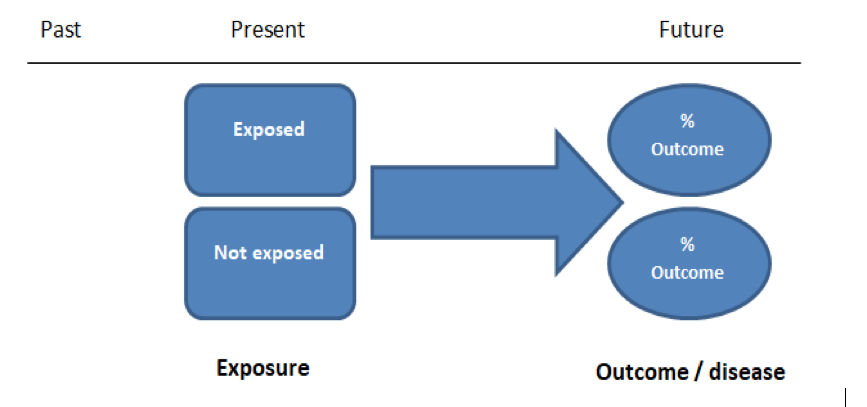



Case Control And Cohort Studies A Brief Overview Students 4 Best Evidence




Converting An Odds Ratio To A Range Of Plausible Relative Risks For Better Communication Of Research Findings The Bmj




Ppt The Odds Ratio Relative Odds Powerpoint Presentation Free Download Id 6056



What Is The Difference Between The Risk Ratio Rr And The Odds Ratio Or Quora




Definition And Calculation Of Odds Ratio Relative Risk Stomp On Step1




Measures Of Disease Association Ppt Download




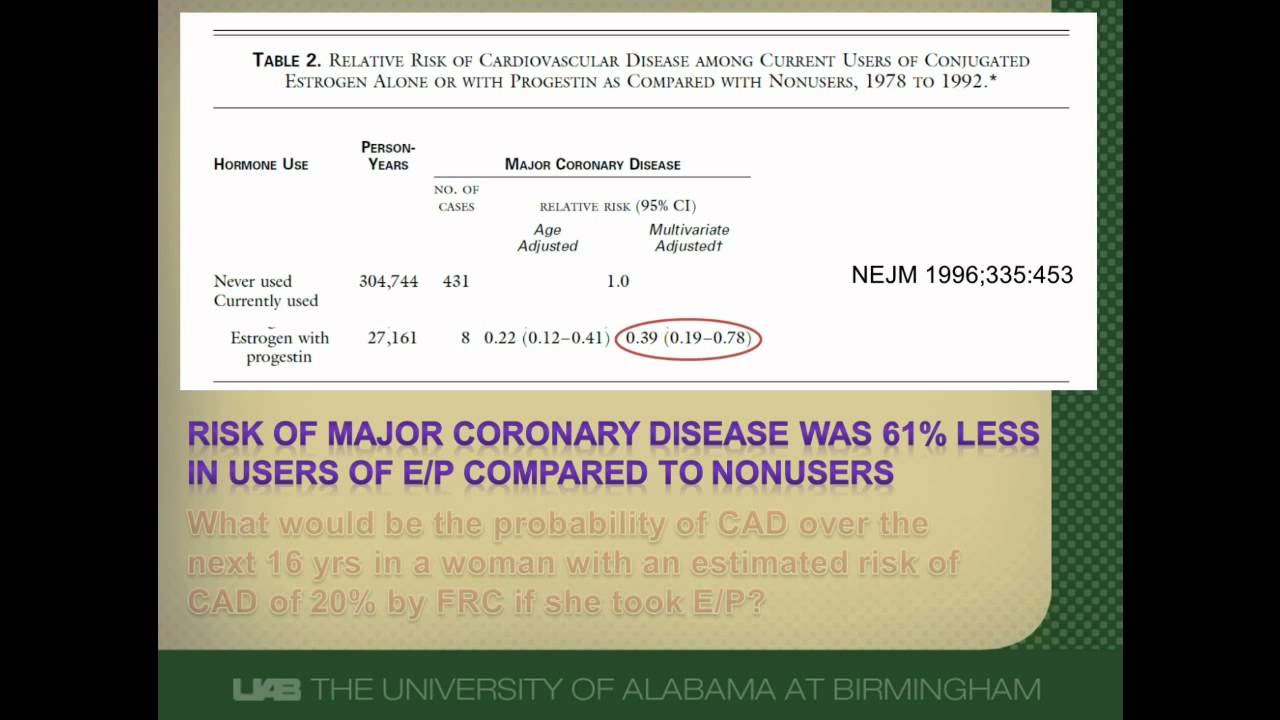
Relative Risk Http Www Slideshare Net Terryshaneyfelt7 What Does An Odds Ratio Or Relative Risk Mean Study Skills Statistics Math Research Methods



What Is The Difference Between The Risk Ratio Rr And The Odds Ratio Or Quora



Relative And Attributable Risks Absolute Risk Involves People




Chapter 6 Choosing Effect Measures And Computing Estimates Of Effect Cochrane Training




Reporting The Results Sage Research Methods




Calculation Of Relative Risks Rr And Odd Ratios Or Download Table



Support Sas Com Resources Papers Proceedings11 345 11 Pdf



0 件のコメント:
コメントを投稿